Why is Ice Less Dense Than Water
All-purpose flour can be. Why cant you use regular all-purpose flour you might ask.

Scientific Explanation Of Why Ice Floats Elementary Science Floating Hydrogen Bond
Part of our research focused on the Vanderford Glacier in East Antarctica.

. When making bread it is very important to use bread flour instead of all-purpose flour. Water is most dense at 39F and as it cools or warms from this temperature the water expands slightly. The floating ice slows the freezing process by insulating the water.
Mathematically density is defined as mass divided by volume. Galileo revealed strange pits and domes that suggest Europas ice layer could be slowly churning or convecting cooler denser ice sinks while warmer less-dense ice rises due to heat from below. This means that ice is slightly less dense than cold water which is why ice floats on the surface of bodies of water.
Diesel is an energy-dense fuel that makes it a great option for semis and large trucks pulling heavy loads. Ceres is more similar to the terrestrial planets Mercury Venus Earth and Mars than its asteroid neighbors but it is much less dense. There are several factors which can affect the density of a substance.
The density of water is approximately 1 gram cubic centimetre 1 gcm 3. Why does ice float on water. These peculiar effects are due to the highly directional bonding of water molecules via the hydrogen bonds.
Water at the Earths surface evaporates into water vapor which rises up into the sky to become part of a cloud which will float off with the winds eventually releasing water back to Earth as precipitation. The other style known as. Since ice is less dense than liquid water it floats in liquid water and prevents the freezing of the whole water body.
As you might expect water density is an important water measurement. One style known in the ice cream trade as Philly-style contains milk cream sugar and flavorings. The less-dense stuff floats on top of the dense liquid.
So lets take a look. There we observed the warm water replacing the colder dense shelf water. Ice is less dense than water.
Ice is less dense than liquid water which is why your ice cubes float in your glass. Ice and liquid water at low temperature have comparatively low-density low-energy open lattice structures. The movement of warm waters towards East Antarctica is expected to worsen throughout the 21st century further threatening the ice sheets stability.
Water currents prevent the ice from sinking. Long linear fractures are often only 1-2 kilometers wide but can extend for thousands of kilometers across Europas surface. The atmosphere is the superhighway in the sky that moves water everywhere over the Earth.
Life can be found in many parts of the geosphere hydrosphere and atmosphere. Water is called the universal solvent because more substances dissolve in water than in any other chemical. Some think this new epoch should start at the Industrial Revolution some at the advent of agriculture 10000 to 15000 years ago.
And to press the veggies through a mesh strainer to get all of the broth and nutrients out. This has to do with the polarity of each water molecule. Due to the high fat content of ice cream however and because fat is less dense than water any ice cream will always be less dense than any aqueous solution otherwise you would not be able to make root beer floats.
In some cases for instance in the. Aquatic life buoys the ice. Ice cream is an emulsiona combination of two liquids that dont normally mix together.
In practical terms density is the weight of a substance for a specific volume. The Anthropocene where anthro means human. This helps water.
I used 4 more cups of water than suggested because 12 cups really wasnt enough to even nearly cover the contents. The density of water once again is a special case. The hydrosphere is the ice water vapor and liquid water in the atmosphere ocean lakes streams soils and groundwater.
Without this property of water life would probably not be possible. The hydrogen side of each water H 2 O molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. The density of water is roughly 1 gram per milliliter but this changes with temperature or if there are substances dissolved in it.
American ice cream comes in two styles. Humans have had such a profound impact on the planets ecosystems and climate that Earth might be defined by a new geological epoch. Some of these fractures have built up into.
Humans are of course part of the biosphere and human activities have important impacts on. Its much more efficient than gasoline but it comes with a premium price tag. Delivered as a flat one-pound sack they absorb up to 45 pounds of fresh water in five minutes forming a dense gel that blocks and redirects water while forming to each other or adjacent structures for a tighter fit than traditional sandbags.
The Great Lakes of the United States are often covered with a sheet of ice every winter. Some factors which affect the density of water are given in the points below. In fact Ceres could be composed of as much as 25.
Imagine how hard would it be to have life in water if every winter the whole water froze. This section of the Water Science School discusses the Earths natural. Water vapor gets into air mainly by evaporation some of the liquid water from the ocean lakes and rivers turns into water vapor and travels in the air.
Why this matters to marine life. -best explains why this ice does not sink to. The water and ice that make up clouds travels into the sky within air as water vapor the gas form of water.
Ceres probably has a solid core and a mantle made of water ice. The presence of living organisms of any type defines the biosphere. Ice is less dense than water.
Factors Affecting Water Density. Where ρ is the density m is the mass and V is the volume. Density volumetric mass density or specific mass is the substances mass per unit of volumeThe symbol most often used for density is ρ the lower case Greek letter rho although the Latin letter D can also be used.
It is the weight of the water per unit volume which depends on the temperature of the water. Regular hexagonal ice is also less dense than liquid waterupon freezing the density of water decreases by about 9. With the increased price in fuel lately many drivers wonder why diesel is more expensive than gas.
In general a solid mass of a substance sinks when immersed in a liquid of the same substance. One of the similarities is a layered interior but Ceres layers arent as clearly defined. The solid form of water ice is less dense than its liquid form.
This feeds into the popular notion that environmental. Solid atoms or molecules are more closely packed together than liquid atoms or. The recipe needs more detail like soaking and rinsing the seaweed whether to cut off roots of onion celery garlic skins etc.
Instead one of the liquids is dispersed throughout the other. Because the ρwater of ice is smaller than the ρwater of water ice floats on water. When air rises in the atmosphere it gets cooler and is under less pressure.
Inflatable sandbag uses a degradable polymer to absorbs water then block it. Well its simple bread flour has an increased amount of protein or more gluten which results in a lighter fluffier dough which produces a less dense finished product.

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Language Biology Chemistry

Ice Floats Because Solid Water Is Less Dense Than Liquid Water Arctic Landscape Landscape Outdoor
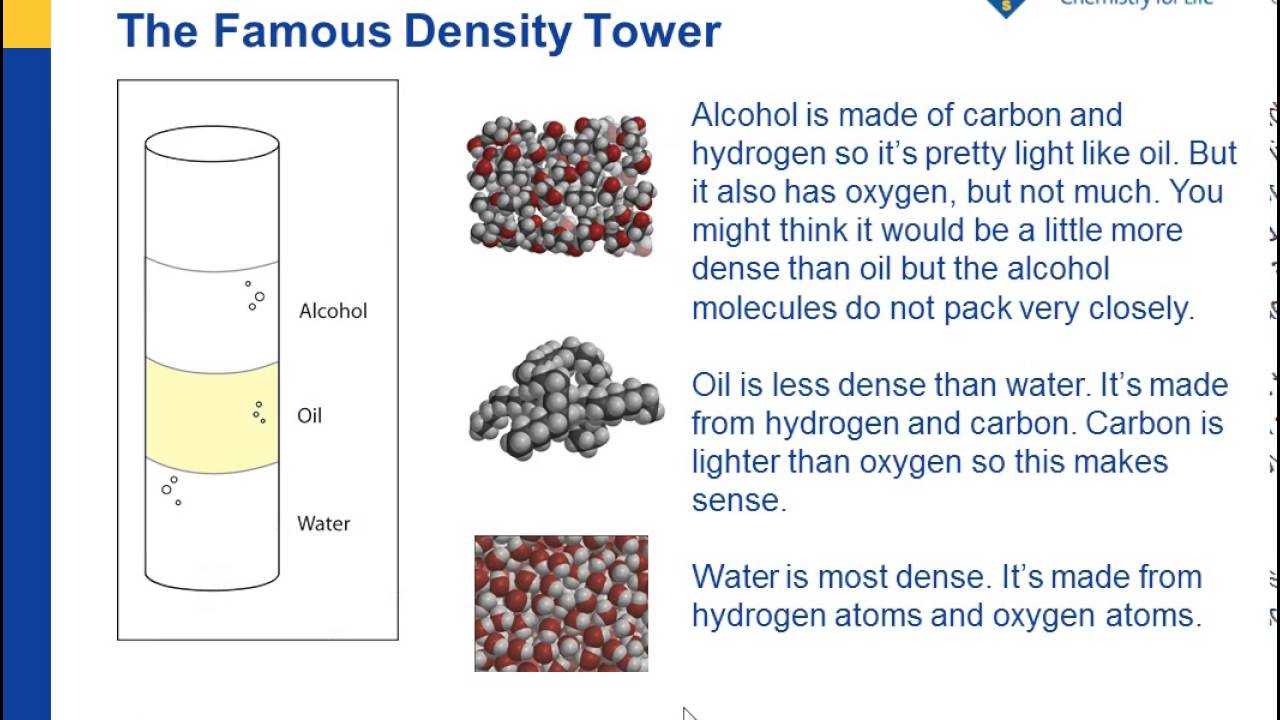
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry Middle School Chemistry Teaching Chemistry Chemistry

Pin By Debashis Panda On Chemistry Hydrogen Bond Water Bond Water Molecule
Comments
Post a Comment